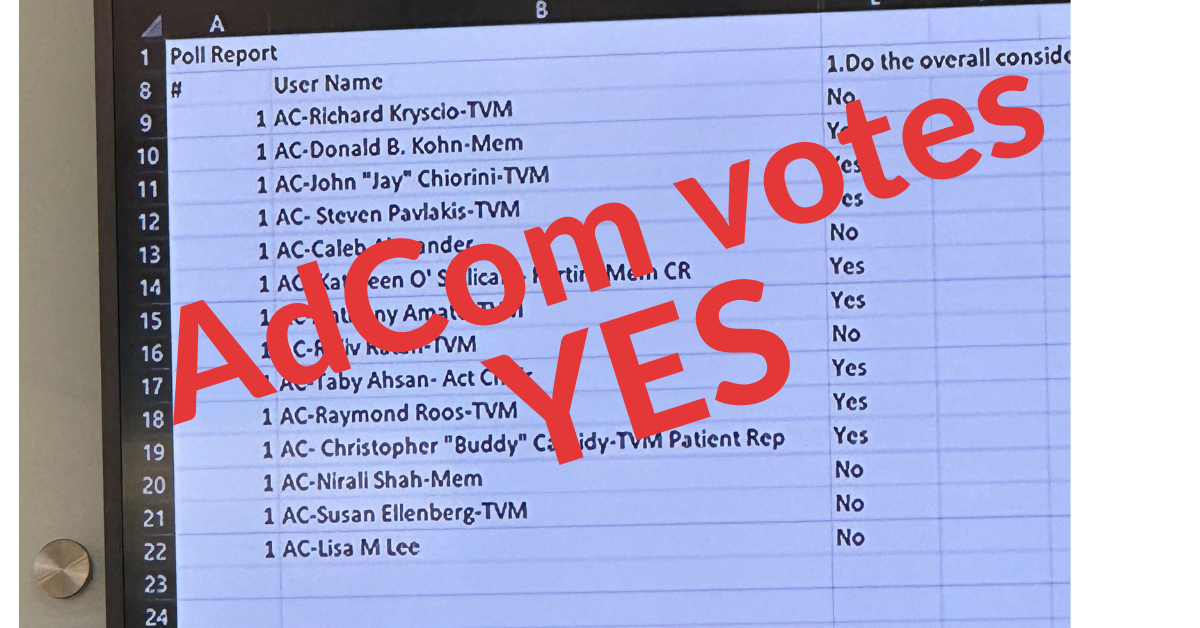
With a mission to cure Duchenne muscular dystrophy
CureDuchenne breaks the traditional charitable mold and balances passion with business acumen. We will fulfill our mission to cure Duchenne muscular dystrophy with our innovative venture philanthropy model that funds groundbreaking research, early diagnosis and treatment access. With pioneering education and support programs, our organization drives real change for those with Duchenne muscular dystrophy and their loved ones.
The CureDuchenne one-to-one program provides meetings with our scientists, physical therapists, Duchenne parents, fundraising team and family support resource coordinators. Email us at Cares@CureDuchenne.org to set up a time that works with your schedule. Together we will cure Duchenne muscular dystrophy!
Making an Impact
Accelerating Curative treatments
10
Since the inception of CureDuchenne, life expectancy for Duchenne has increased by a decade.
Funding Critical Clinical Trials
18
18 projects funded by CureDuchenne have progressed to clinical trials.
Making an Impact
$50+M
We’ve raised over $50 million for research, education and care.
Accelerating a Cure
1st
CureDuchenne contributed early funding for the first FDA-approved Duchenne drug.
Working Towards the Future
$3B
Our model has leveraged over $3 billion in follow-on funding for future programs from investors and biotech companies.

Duchenne is a devastating muscle disease.
Duchenne muscular dystrophy (DMD) is the most common form of muscular dystrophy, occurring in approximately 1:5,000 male births. Those affected with DMD lose their ability to walk, feed themselves, breathe independently and succumb to heart failure.
But there’s hope through new pharmacological and gene-based therapies. You can help make a difference in finding a cure.